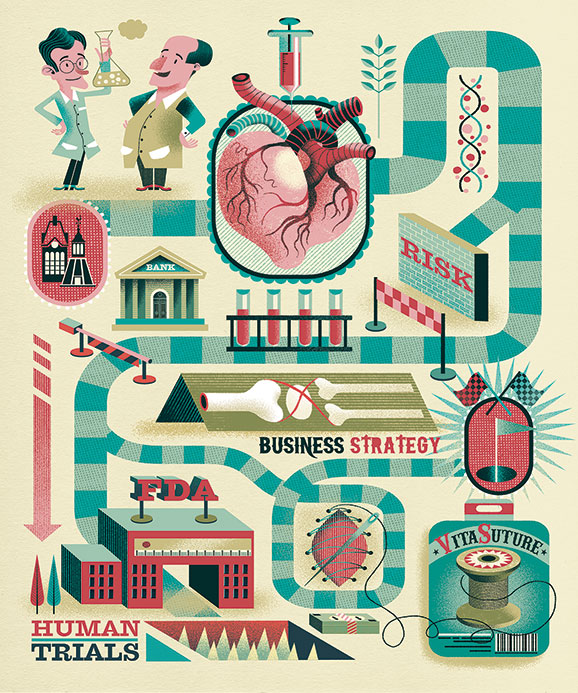
 WPI researchers are known for taking on real-world problems, which means the discoveries and innovations they create in the lab often have potential value in the marketplace. But when scientists and engineers decide to take the leap and become entrepreneurs, they can encounter unexpected twists, turns, and detours on the road to commercialization. Navigating such an uncertain path requires creative pivots in strategy. And it’s not uncommon for the product that makes it to market to be significantly different from the inventor’s original vision. That, in a nutshell, is the story of two WPI biomedical engineers with innovative medical technology and a dream of saving lives. Buckle your seatbelts as we retrace their eye-opening journey.
WPI researchers are known for taking on real-world problems, which means the discoveries and innovations they create in the lab often have potential value in the marketplace. But when scientists and engineers decide to take the leap and become entrepreneurs, they can encounter unexpected twists, turns, and detours on the road to commercialization. Navigating such an uncertain path requires creative pivots in strategy. And it’s not uncommon for the product that makes it to market to be significantly different from the inventor’s original vision. That, in a nutshell, is the story of two WPI biomedical engineers with innovative medical technology and a dream of saving lives. Buckle your seatbelts as we retrace their eye-opening journey.
IT SOUNDED LIKE A PERFECT PLAN
VitaThreads, a spin-off founded in 2012 by Professor Glenn Gaudette, PhD, and Associate Professor George Pins, PhD, of biomedical engineering, started as a perfect partnership of people and ideas. Pins patented microthreads made from fibrin and other biological polymers that have the ability to stimulate the repair of injured tissue. Gaudette, who explores methods for regenerating damaged heart muscle using stem cells, had begun experimenting with the use of the micro-threads to deliver stem cells to the heart. With Pins and Marsha Rolle, PhD, associate professor of biomedical engineering, he came up with the notion of growing human mesenchymal stem cells (hMSCs) directly on the threads and stitching them into the myocardium. The idea seemed to offer new hope to heart attack victims.
Subsequent experiments showed that the threads could deliver stem cells where they’re needed and get them to stay put. Stem cell therapy holds great promise for promoting tissue regeneration. But in the heart, the “delivery dilemma” is a significant obstacle. Other means of introducing the cells — such as injecting them intramuscularly or sewing seeded “patches” over the damaged area — are risky and unreliable. Only about 10 percent of the stem cells embed, and few of those survive. But in animal studies, about 60 percent of the cells delivered via microthread sutures took hold.
Despite that success, the heart would prove to be the wrong place for this small, inexperienced company to start its journey. “The heart is a risky organ,” Gaudette says, “and this is still a relatively invasive procedure. If you mess up in the heart, it can be a life or death matter.” The researchers reached out to VitaThread’s board of directors and its CEO Harry Wotton ’94 — a successful biomedical entrepreneur — as well as the team assembled to help guide them by WPI’s Tech Advisors Network Their advice? Come up with an initial application with a lower risk barrier.
Gaudette recalls, “At the time, a friend of ours had ruptured his Achilles tendon. He had to stay off it for six months. That’s a long healing process, and function is not always fully restored. That got us thinking about a new tack.”
In previous work, Pins had demonstrated that the micro-threads, all by themselves, could be bundled into cables and used as scaffolds to accelerate the repair of ligaments and tendons. His initial target was the knee’s ACL (anterior cruciate ligament). Adding stem cells would enhance the threads’ benefits, Pins and Gaudette theorized, since they’d promote the growth of new connective tissue. While animal studies were quite promising, the researchers hit a roadblock when they started exploring the potential for human trials. “The regulatory hurdles for using stem cells in humans are not trivial,” Gaudette says.
When they presented their concept to their FDA regulatory advisor, his curt reaction cut to bone: “Love the sutures,” he said, “but if you want to get through approvals quickly, lose the stem cells.” He advised them to strip their medical miracle down to their MVP — minimum viable product — a business strategy that involves rolling out the most basic form of a technology and leaving more advanced features for later. For VitaThreads, the MVP was the unadorned microthread suture. The group that had been advising Gaudette and Pins suggested they shoot for an equally simple initial application: stitching up skin.
The blow hit Gaudette the hardest. “Compared to operating on a beating, contracting heart, repairing a tendon is basically just sewing a rope together,” he says. “It’s still interesting, but to go from that to stitching up skin...,” he pauses, the disappointment audible in his voice. “I’m a cardiac guy; I cut through the skin to get to the heart. When I’m done, I let my students close up.”
“He’s gone from repairing Lamborghinis to fixing lawnmowers,” Pins teases.
Accepting the realities of the regulatory landscape, VitaThreads settled on its first application. Instead of enabling surgeons to save the lives of heart attack victims or helping athletes heal faster from devastating injuries, their product, now known as the VitaSuture Wound Management System (VWMS), would help plastic surgeons complete face lifts, nose jobs, and eyelid revisions with a much-reduced chance of high-visibility scars. With more than seven million reconstructive and elective facial surgeries performed annually in the United States, it is a sizable market.
In July 2014, the company reported on the results of its pre-clinical in vivo studies. When used with a rat skin incisional model, the sutures were completely absorbed within 14 days, about half the time required for conventional absorbable sutures, and with reduced inflammation. For human patients, those results promised faster recovery with fewer complications and a better cosmetic outcome.
“The heart is a risky organ and this is still a relatively invasive procedure. If you mess up in the heart, it can be a life or death matter.”
Glenn Gaudette, PhD
Professor, Biomedical Engineering

 Inventors don’t stand alone at WPI. The spinoff of VitaThreads got a leg up from two initiatives designed to help university researchers become entrepreneurs.
Inventors don’t stand alone at WPI. The spinoff of VitaThreads got a leg up from two initiatives designed to help university researchers become entrepreneurs.
