Amyloid polypeptides are short chains of amino acids that have been linked to a variety of diseases, including Alzheimer’s and Parkinson’s. Their tendency to aggregate into fibers leads to the disruption of the normal functioning of cells and organs. In a recent paper published in the journal Langmuir ("Influence of the Human and Rat Islet Amyloid Polypeptides on Structure of Phospholipid Bilayers: Neutron Reflectometry and Fluorescence Microscopy Studies"), WPI physicist Izabela Stroe, PhD, in collaboration with colleagues at Los Alamos National Laboratory, the University of California Davis, and Yale University, reported on discoveries that shed new light on the role of amyloid polypeptides in type 2 diabetes, the seventh leading cause of death in the United States.
Amyloid polypeptides are short chains of amino acids that have been linked to a variety of diseases, including Alzheimer’s and Parkinson’s. Their tendency to aggregate into fibers leads to the disruption of the normal functioning of cells and organs. In a recent paper published in the journal Langmuir ("Influence of the Human and Rat Islet Amyloid Polypeptides on Structure of Phospholipid Bilayers: Neutron Reflectometry and Fluorescence Microscopy Studies"), WPI physicist Izabela Stroe, PhD, in collaboration with colleagues at Los Alamos National Laboratory, the University of California Davis, and Yale University, reported on discoveries that shed new light on the role of amyloid polypeptides in type 2 diabetes, the seventh leading cause of death in the United States.
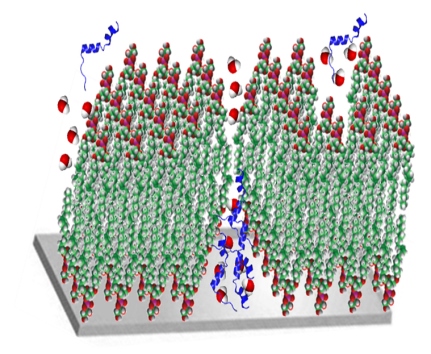
While the genesis of diabetes remains unknown, it is clear that the disease occurs when cells in the periphery of the body become less sensitive to insulin. Beta cells in the pancreas then step up their production of the hormone, which regulates blood glucose. Along with insulin, the beta cells produce islet amyloid polypeptide (IAPP). As insulin production increases, the excess amyloid molecules begin aggregating into fibers. Somewhere along the way, the beta cells begin to die (leading to insulin deficiency) through an as yet unknown mechanism that appears to involve damage to the cell membranes.